Iron and Steel Smelting: The Transformation Journey from Ore to Steel
Iron and steel, known as the "skeleton" of modern industry, underpins the development of numerous fields such as construction, automotive, machinery, and aerospace. Its creation is not an overnight achievement but a series of complex and sophisticated smelting processes. Starting from iron ore lying dormant in the Earth's crust, it gradually transforms into high-quality steel that possesses both strength and toughness. This process mainly consists of five core stages: raw material preparation, blast furnace ironmaking, converter steelmaking, secondary refining, and continuous casting. Each stage embodies humanity's continuous exploration and breakthroughs in metallurgical technology.
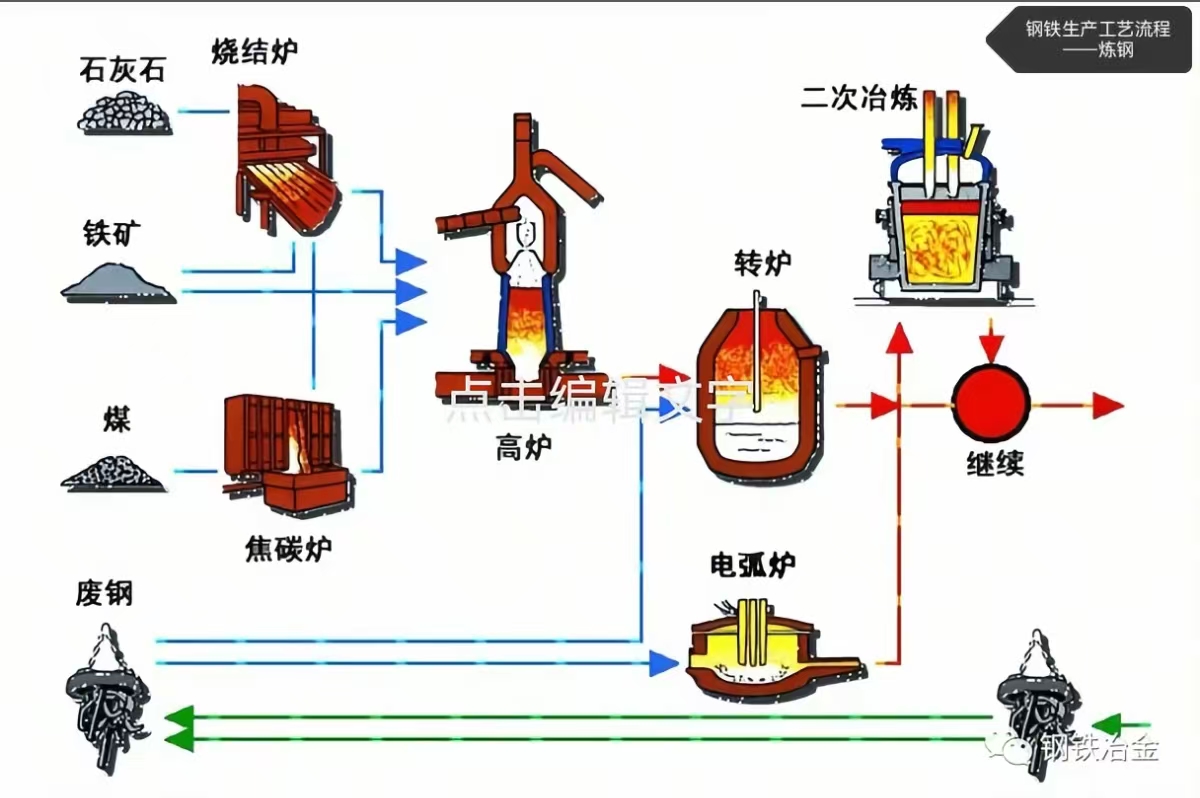
1. Raw Material Preparation: Laying the Foundation for Smelting
The first step in iron and steel smelting is the strict pre-treatment of various raw materials to ensure their composition, particle size, and purity meet the requirements of subsequent processes. The raw materials at this stage mainly include iron ore, coke, limestone, and scrap steel (used in the steelmaking stage), with clear processing standards for each type.
Natural iron ore often contains a large amount of impurities (such as gangue, sulfur, phosphorus, etc.). Therefore, it first needs to be processed into uniformly sized ore powder using crushing and grinding equipment. Then, mineral processing technologies like magnetic separation and flotation are employed to remove impurities, increasing the iron content (iron grade) to over 60% to produce "iron concentrate." Some iron concentrates are further pressed into "pellets" or mixed with coal powder to make "sinter." These two forms of ore have better air permeability, enabling full reaction with coke in the blast furnace and improving ironmaking efficiency.
Coke is the core fuel and reducing agent in blast furnace ironmaking. It is produced by high-temperature carbonization of coking coal in a coke oven under air-isolated conditions. During the coking process, volatile components in the coal are removed, forming coke with well-developed pores and high strength. Its main functions are to provide heat through combustion (maintaining a high temperature of around 1500°C inside the blast furnace) and reduce iron oxides in the iron ore. In addition, limestone, acting as a "flux," needs to be crushed into an appropriate particle size. It reacts with gangue in the blast furnace to form slag with a lower melting point, facilitating separation from molten iron.
2. Blast Furnace Ironmaking: "Extracting" Molten Iron from Ore
Blast furnace ironmaking is the key stage for reducing iron elements in iron ore into liquid iron (molten iron). Its core equipment is a blast furnace tens of meters high. A modern blast furnace can produce thousands of tons of molten iron per day, earning it the title of the "heart" of a steel plant.
At the start of the ironmaking process, workers mix pre-treated iron ore (sinter or pellets), coke, and limestone in a certain proportion, and feed them into the furnace in batches through the bell or bell-less top device at the top of the blast furnace, forming layered "furnace charge." Meanwhile, the tuyeres at the bottom of the blast furnace blow hot air (heated to over 1000°C by a hot blast stove using blast furnace gas combustion) into the furnace. The hot air reacts rapidly with coke to generate carbon monoxide (CO) and hydrogen (H₂), releasing a large amount of heat and gradually increasing the temperature inside the furnace.
In the high-temperature environment, carbon monoxide and hydrogen act as reducing agents, undergoing chemical reactions with iron oxides (such as iron oxide and magnetite) in the iron ore to reduce iron elements, forming liquid "molten iron." The gangue (such as silica) in the iron ore reacts with limestone to form slag with a melting point of approximately 1400°C. Since the density of slag is lower than that of molten iron, it floats on the surface of the molten iron, protecting the molten iron from oxidation.
When a certain amount of molten iron and slag accumulates in the furnace, the taphole and slag hole at the bottom of the blast furnace are opened in sequence. The molten iron flows through the iron runner into the molten iron ladle and is transported to the steelmaking workshop; the slag is discharged through the slag runner, and after cooling, it can be used to make cement, roadbed materials, etc., realizing resource recycling. It is worth noting that a large amount of blast furnace gas (mainly composed of carbon monoxide, nitrogen, and hydrogen) is also produced during blast furnace ironmaking. After purification, it can be used as fuel for hot blast stoves and coke ovens, reducing energy consumption.
3. Converter Steelmaking: Removing Impurities and Adjusting Composition
The molten iron produced by the blast furnace has a relatively high carbon content (generally 3.5%-5%) and contains impurities such as silicon, manganese, sulfur, and phosphorus. It is brittle and cannot be directly used for manufacturing steel products. Therefore, the steelmaking stage is required to reduce the carbon content, remove impurities, and adjust the proportion of alloying elements to meet the performance requirements of the desired steel. Currently, the most widely used steelmaking process in industry is converter steelmaking (mainly oxygen top-blown converters), which is characterized by fast smelting speed, high efficiency, and low cost.
The core equipment of converter steelmaking is a pear-shaped converter. The furnace body can tilt around the horizontal axis, facilitating charging, steelmaking, and tapping. Before steelmaking begins, the converter is tilted at a certain angle, and workers pour the molten iron (at a temperature of approximately 1300°C) transported from the blast furnace into the furnace. At the same time, an appropriate amount of scrap steel (used to adjust the temperature of the molten iron and reduce costs, generally accounting for 10%-30% of the total furnace charge) and lime (used as a slagging agent to remove sulfur and phosphorus impurities) are added.
Subsequently, the converter is erected, and the oxygen lance at the top is inserted into the furnace, blowing high-pressure oxygen with a purity of over 99.5% (oxygen pressure can reach 0.8-1.2MPa) into the molten iron. Oxygen reacts violently with elements such as carbon, silicon, and manganese in the molten iron: carbon reacts with oxygen to form carbon monoxide (some is burned into carbon dioxide), and silicon and manganese react with oxygen to form silicon dioxide and manganese oxide. These oxides then react with lime to form slag. A large amount of heat released during the reaction process (such as the oxidation reaction of carbon) rapidly raises the temperature inside the furnace to 1600-1700°C, ensuring that the molten iron is completely in a liquid state and impurities react fully.
During the steelmaking process, workers monitor the carbon content and impurity content of the molten steel in real-time through sampling analysis or online detection (such as spectral analysis). When the composition meets the target requirements, oxygen blowing is stopped, the converter is tilted, the upper layer of slag is discharged first, and then the molten steel is poured into the ladle and sent to the secondary refining stage. The entire converter steelmaking process usually takes only 20-40 minutes, and a 300-ton converter can produce approximately 300 tons of molten steel per heat.
4. Secondary Refining: "Fine Processing" to Improve Molten Steel Quality
With the increasing requirements of modern industry for steel quality (such as high-strength steel, corrosion-resistant steel, and other special steels), converter steelmaking alone can hardly meet the requirements of precise composition control and purity. Therefore, a secondary refining stage is needed to perform further "fine processing" on the molten steel. The main functions of secondary refining are: adjusting the composition of molten steel (precisely controlling the content of carbon and alloying elements), removing gases (such as hydrogen and nitrogen) and inclusions (such as oxides and sulfides) in the molten steel, and homogenizing the temperature of the molten steel.
Currently, commonly used secondary refining equipment includes LF (Ladle Furnace), RH (Ruhrstahl-Heraeus) vacuum refining furnace, VD (Vacuum Degassing) furnace, etc., with each type of equipment having different focuses on functions. Taking the most widely used LF as an example, its core is to heat the molten steel through electrodes to maintain the temperature of the molten steel (preventing solidification), and at the same time, add alloy materials (such as ferromanganese, ferrosilicon, ferrochrome, etc.) to the molten steel to precisely adjust the composition of the steel, making it meet the performance requirements of different steels (for example, high-strength steel needs to add alloying elements such as vanadium and niobium).
The RH vacuum refining furnace is mainly used to remove gases from the molten steel: the molten steel is sucked into a vacuum chamber. In a vacuum environment, the solubility of gases such as hydrogen and nitrogen in the molten steel decreases, and they escape in the form of bubbles, thereby significantly reducing the gas content in the molten steel (for example, the hydrogen content can be reduced to below 2ppm). This avoids defects such as bubbles and cracks in the steel during subsequent processing or use. In addition, during the secondary refining process, refining slag is added and the molten steel is stirred to further remove inclusions in the molten steel and improve the purity of the molten steel.
5. Continuous Casting: Converting Molten Steel into Steel Billets
The molten steel after secondary refining finally needs to be converted into steel billets of fixed shapes (such as slabs, blooms, billets, etc.) through the continuous casting process, preparing for the subsequent steel rolling stage (rolling the steel billets into finished steel products such as steel plates, steel pipes, and section steels). The emergence of the continuous casting process has replaced the traditional "ingot casting" (pouring molten steel into casting molds to cool into steel ingots), greatly improving production efficiency and reducing energy consumption and metal loss.
The core equipment of continuous casting is the continuous caster, which mainly consists of a mold, secondary cooling zone, withdrawal and straightening unit, cutting equipment, etc. The process is as follows: first, the refined molten steel (at a temperature of approximately 1500°C) is transported to the turret of the continuous caster via a ladle, and then the tundish (which plays a role in stabilizing the molten steel flow and distributing the molten steel) uniformly injects the molten steel into the mold below. The mold is a copper-made die with cooling water flowing inside (its shape is consistent with the desired steel billet, such as rectangular, circular, etc.). When the molten steel comes into contact with the cooling water in the mold, its surface cools and solidifies rapidly, forming a solid billet shell with a thickness of about 10-20mm, while the interior remains liquid.
Subsequently, the withdrawal and straightening unit below the mold pulls the initially solidified steel billet out of the mold at a constant speed (generally 0.5-2m/min) and enters the secondary cooling zone. In the secondary cooling zone, a large amount of cooling water (sprayed through nozzles) is sprayed onto the surface of the steel billet, allowing the liquid molten steel inside the steel billet to gradually cool and solidify, forming a fully solid steel billet. Finally, when the steel billet is pulled to a certain length, the cutting equipment (such as a flame cutter or plasma cutter) cuts it into fixed-length steel billets (generally 6-12m in length). After passing inspection, these steel billets are sent to the steel rolling mill, and through processes such as rolling and heat treatment, they eventually become various finished steel products.
Conclusion
From the pre-treatment of iron ore to the production of steel billets, iron and steel smelting goes through five core stages: raw material preparation, blast furnace ironmaking, converter steelmaking, secondary refining, and continuous casting. Each stage relies on advanced equipment, precise control, and professional technology. With the development of science and technology, iron and steel smelting technology is also constantly advancing - the promotion of short-process steelmaking (using scrap steel as raw material and steelmaking via electric arc furnaces), the application of intelligent control systems, and the research and development of green and low-carbon technologies (such as hydrogen-rich ironmaking and carbon dioxide capture) are driving the iron and steel industry towards a more efficient, environmentally friendly, and high-quality direction. As the cornerstone of modern industry, the iron and steel smelting process is not only a material transformation "from ore to steel" but also a vivid testimony to the continuous progress of human industrial civilization.
